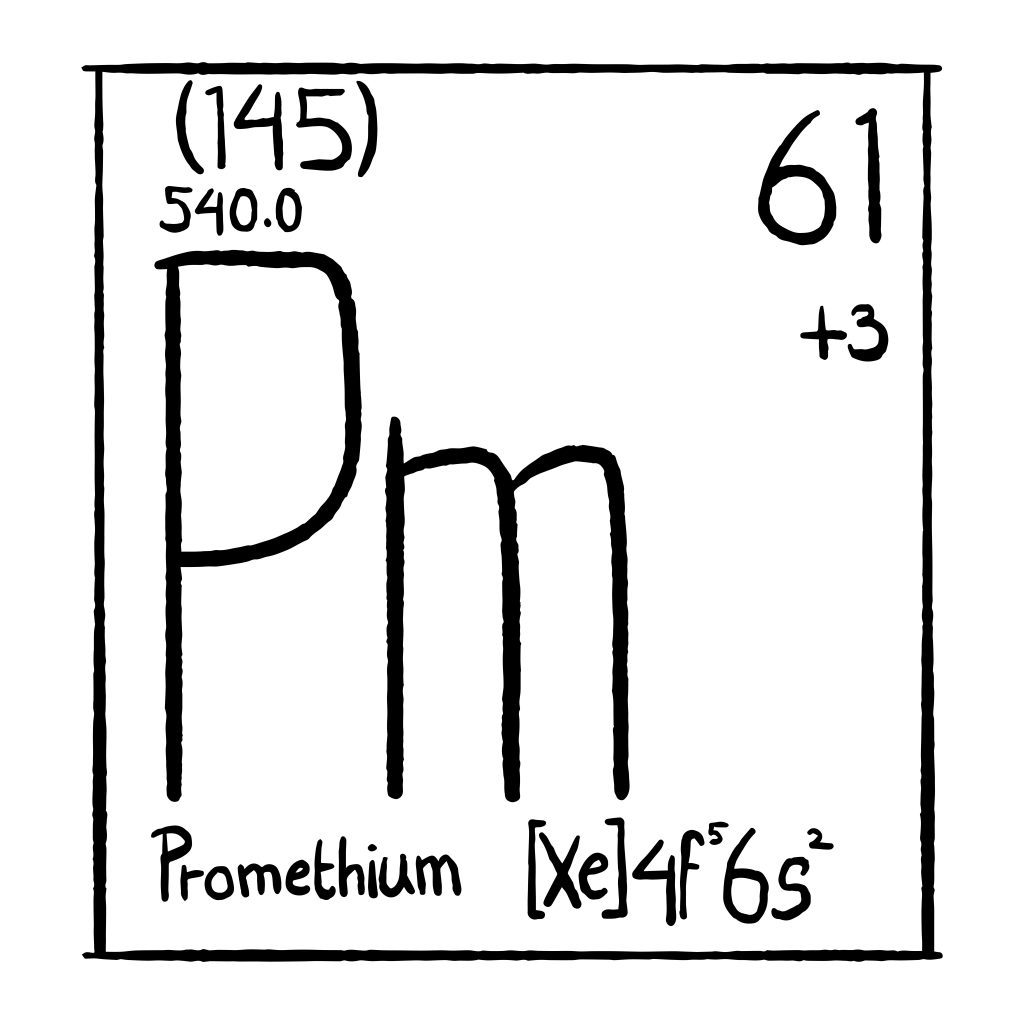
Promethium is a chemical element with the atomic number 61, making it part of the lanthanide series in the periodic table. It is classified as a rare earth metal, located between neodymium (atomic number 60) and samarium (atomic number 62). Unlike most elements in the periodic table, Promethium is not found naturally in significant amounts on Earth. It is primarily produced synthetically in nuclear reactors or as a byproduct of the decay of uranium and thorium.
Importance of Promethium
Promethium is a particularly interesting element due to several key features. Firstly, it is radioactive, with no stable isotopes existing in nature. This characteristic makes it unique among the rare earth metals, as it is the only one that lacks a stable form. Additionally, because of its rarity and radioactive properties, Promethium has intriguing applications in various fields, including nuclear batteries, medical imaging, and research. Its scarcity and the difficulty in obtaining it make it a valuable subject of study, enhancing its significance within both the scientific community and the broader realm of materials science.
What is Promethium?
Promethium is a radioactive chemical element with the atomic number 61. It is classified as a rare earth metal, positioned in the lanthanide series of the periodic table. This series includes elements that share similar properties, such as high melting points and strong magnetic behavior. However, Promethium is distinct in that it is the only rare earth metal that does not have a stable isotope, making it an especially unique element among its peers. Due to its radioactive nature, Promethium is primarily produced synthetically in nuclear reactors, and it is not found in significant natural deposits on Earth. The element has a silvery-white appearance and is relatively unstable, decaying into other elements over time, which is one of the reasons it is so rare in nature.
Discovery of Promethium
The discovery of Promethium is relatively recent in the history of chemistry, taking place in 1945 at the University of Chicago. The element was identified by American scientists Jacob Marinsky, Lawrence Glendenin, and Charles Coryell. Their work followed years of speculation about the existence of an element between neodymium and samarium, which was thought to be missing from the periodic table. The researchers isolated Promethium from uranium fission products, confirming its presence and marking it as a new element. The name „Promethium” was chosen in honor of the Greek mythological figure Prometheus, who defied the gods by giving fire to humanity. This symbolic connection reflects the element’s somewhat elusive and powerful nature, as Promethium’s discovery was a monumental breakthrough in the understanding of nuclear chemistry and radioactive elements.
Position in the Periodic Table
Promethium occupies a unique position in the periodic table as part of the lanthanide series, which consists of 15 elements that range from lanthanum to lutetium. These elements are typically found in the f-block of the periodic table and share common characteristics, such as high magnetic susceptibility and the ability to form various oxidation states. Promethium’s classification as a rare earth metal is due to its scarcity in nature, as it is rarely found in significant quantities outside of laboratory settings. Its properties further set it apart: Promethium is a silvery-white metal that is highly radioactive and relatively unstable. Unlike most other elements, which have stable isotopes, Promethium’s isotopes decay, adding to its rarity and intrigue. This combination of radioactive behavior and its distinct place in the lanthanide series makes Promethium a fascinating subject of study within nuclear chemistry and materials science
Atomic Structure
Promethium, with the atomic number 61, occupies a unique position on the periodic table as a lanthanide element. Its electron configuration is [Xe] 4f^5 6s^2, which means that in its ground state, Promethium has five electrons in the 4f orbital, characteristic of elements in the lanthanide series. This arrangement of electrons contributes to Promethium’s distinctive chemical properties, including its ability to form various oxidation states, typically +3. Promethium has no stable isotopes, which is one of its most notable characteristics. The most stable isotope of Promethium is Promethium-145, which has a half-life of around 17.7 years, but all of its isotopes are radioactive and decay over time. This lack of stable isotopes is a defining feature of the element, contributing to its rarity and the challenges associated with studying it.
Radioactivity
The radioactive nature of Promethium is one of its most defining characteristics. Because Promethium lacks stable isotopes, it exists only in radioactive forms that decay into other elements over time. This radioactivity makes it valuable in specific applications, such as nuclear batteries and medical imaging, but also means that Promethium is highly unstable and difficult to work with in larger quantities. The isotopes of Promethium emit beta radiation as they decay, and the element itself can be produced artificially through nuclear reactions, such as those occurring in reactors or particle accelerators. Promethium’s radioactivity not only makes it fascinating from a scientific standpoint but also contributes to its scarcity in nature, as the element’s short-lived isotopes quickly decay into other elements, preventing the accumulation of substantial natural deposits.
Physical Properties
Promethium is a silvery-white metal that shares many physical properties with other lanthanides. As a relatively soft metal, it has a moderate melting point of approximately 1,042°C and a boiling point around 3,000°C, indicating it can withstand relatively high temperatures before changing states. Its density is about 7.26 g/cm³, which places it in the range of other rare earth metals. Though it is not as well-known as some other metals, Promethium does possess interesting magnetic and electrical properties. It is paramagnetic, meaning it is weakly attracted by magnetic fields, a feature common among many rare earth metals. Additionally, Promethium exhibits electrical conductivity, though not as high as metals like copper or silver, which is typical for lanthanide elements. The combination of its silvery-white appearance, moderate density, and unique magnetic properties makes Promethium a remarkable, albeit rare, element in both theoretical and practical contexts
Overview of Isotopes
Promethium has several isotopes, all of which are radioactive. These isotopes differ in the number of neutrons in their nuclei, leading to variations in their stability and half-lives. Promethium-145 (Pm-145) and Promethium-147 (Pm-147) are the most stable isotopes of this element, although they are still radioactive. Pm-145 has a half-life of around 17.7 years, while Pm-147 is more commonly used due to its relatively longer half-life of 2.62 years. The remaining isotopes of Promethium are less stable, decaying into other elements more quickly, which contributes to the rarity of Promethium in nature.
Half-Life and Applications
The half-life of Promethium isotopes plays a crucial role in their practical applications. Pm-145, with its longer half-life, is useful in certain scientific and industrial contexts, though Pm-147 is the more widely applied isotope. Pm-147, because of its half-life of 2.62 years, is commonly used in applications that require a steady, but not excessive, source of radiation over a period of time. One of the most significant uses of Pm-147 is in radioisotope thermoelectric generators (RTGs), which are employed to power spacecraft and remote weather stations. These generators utilize the heat produced by the decay of radioactive isotopes to generate electricity, making them vital for long-duration space missions, where solar power is not feasible.
Promethium isotopes also have medical applications. Pm-147 is used in certain types of radiation therapy, where it is employed in the form of small sources for cancer treatment, particularly in the treatment of eye cancers or other localized tumors. Additionally, Promethium is used in radioluminescent paint, often in the production of dials, gauges, and other items that require illumination without an external power source. Despite their radioactive nature, the relatively controlled half-lives of Promethium isotopes make them valuable in these applications, where their decay can be harnessed to generate energy or aid in medical treatments without significant risk over time
Natural Occurrence
Promethium is one of the few elements that is not found in significant amounts in nature. Unlike many other elements that occur abundantly in Earth’s crust, Promethium is extremely rare in its natural form. This is primarily due to its radioactive nature; the isotopes of Promethium decay quickly, preventing them from accumulating in significant quantities over time. While trace amounts of Promethium can be found in uranium ores, it is present only in very small quantities as a result of the decay of uranium and thorium. In fact, the natural occurrence of Promethium is so limited that it is almost exclusively produced synthetically, making it a rare and valuable element for scientific and industrial purposes.
Production
Promethium is typically obtained through nuclear reactions in laboratories or nuclear reactors. In these controlled environments, it is often created as a byproduct of the fission of uranium or thorium. When uranium or thorium undergoes nuclear reactions, they can produce various radioactive elements, including Promethium. One common method of producing Promethium involves irradiating uranium with neutrons in a nuclear reactor. This process leads to the formation of Promethium isotopes, particularly Pm-147, which is one of the most stable and useful isotopes for practical applications.
Given the scarcity of naturally occurring Promethium, its synthetic production has made it accessible for use in a range of specialized fields. Although the element is not found in large amounts on Earth, it can be produced in sufficient quantities for use in technologies such as radioisotope thermoelectric generators (RTGs) and in medical treatments. However, the element’s rarity and the need for high-energy processes to create it mean that Promethium remains relatively expensive and limited in supply, reinforcing its status as one of the more intriguing and valuable elements in the periodic table.
Power Sources
One of the most important uses of Promethium, particularly its isotope Promethium-147 (Pm-147), is in power sources. Pm-147 is used in small, long-lasting batteries that provide a steady supply of energy through its radioactive decay. These batteries are particularly valuable in medical devices, such as pacemakers, where reliable power is essential for extended periods without the need for frequent replacements. The radioactive decay of Pm-147 generates heat, which is then converted into electricity through thermoelectric materials, offering a compact and efficient power source for critical applications.
Additionally, Promethium-147 plays a crucial role in space exploration. It is used in radioisotope thermoelectric generators (RTGs), which are devices that convert the heat from the radioactive decay of isotopes into electrical power. RTGs have been a reliable power source for spacecraft and satellites, particularly in missions where solar energy is insufficient. Notable missions such as the Voyager probes and the Mars rovers have used RTGs powered by isotopes like Promethium-147 to operate their instruments and communicate with Earth over extended periods, even in the harsh conditions of deep space, where sunlight is too weak to provide adequate energy.
Other Applications
Promethium also has various applications in other industries, notably in the field of lighting and display technology. It is used in phosphorescent materials, which can absorb energy and re-emit it as light. These materials are applied in certain types of lighting, such as in dials, clocks, and watches, where they provide a glowing effect in low-light conditions without needing an external power source. The radioactive properties of Promethium allow it to glow for extended periods, making it useful in applications requiring long-term illumination.
In the realm of nuclear medicine, Promethium has potential uses, especially in radiation therapy for cancer treatment. Though it is not as widely used as other isotopes like cobalt-60 or iodine-131, Promethium’s radioactive isotopes, particularly Pm-147, could be employed in targeted radiation therapy. The ability to generate radiation on demand makes Promethium a valuable candidate for localized treatments, where controlled doses of radiation can be used to target cancer cells. Its use in medical applications, however, is limited due to its rarity and the challenges associated with handling radioactive materials. Nonetheless, Promethium’s unique properties open up possibilities for its application in specialized fields like medicine and advanced technology.
Handling Radioactivity
One of the primary challenges associated with Promethium is its radioactive nature. As a radioactive element, Promethium, particularly its isotopes like Pm-147, poses potential health risks if not handled with proper precautions. Prolonged exposure to Promethium’s radiation, especially in concentrated amounts, can cause damage to living tissues and increase the risk of cancer. Therefore, strict safety protocols must be followed when working with Promethium in laboratory or industrial settings. Workers must wear protective gear, including lead shielding or other radiation-blocking materials, to minimize exposure. Additionally, specialized equipment is required to safely handle Promethium and other radioactive elements, including sealed containers and radiation detection tools. The controlled environments where Promethium is used—such as nuclear reactors or medical facilities—are designed to ensure that radiation levels remain within safe limits to protect human health.
Waste Management
Another significant challenge related to Promethium is the disposal and management of radioactive waste. Since Promethium is radioactive and decays over time, it contributes to the accumulation of nuclear waste, which must be carefully managed to prevent environmental contamination. Radioactive waste poses a long-term risk because it can remain hazardous for many years, and managing this waste requires specialized storage solutions. In the case of Promethium, its isotopes decay into other radioactive elements, creating a chain of waste that must be securely contained to avoid leakage into the environment. Additionally, because Promethium is often produced in small quantities and used in specific applications, waste disposal methods must be tailored to these circumstances to ensure minimal environmental impact.
To mitigate these risks, strict guidelines and regulations govern the handling and disposal of radioactive materials. Waste must be stored in secure, high-tech facilities that can contain radiation and prevent contamination of the surrounding ecosystem. In some cases, the materials are sealed in containers made from materials such as glass or stainless steel, designed to withstand radiation and isolate the waste for thousands of years. Despite these precautions, the potential environmental impact of improperly managed radioactive waste remains a serious concern, particularly in the event of leaks or accidents. As Promethium and other radioactive materials continue to be used in various applications, the challenges of waste management and ensuring safety remain critical components of their use in industry and research.
Research and Technological Advancements
Ongoing research into Promethium continues to focus on understanding its properties and discovering new ways to utilize its unique characteristics. One area of active research is the development of more efficient methods for producing and storing Promethium, particularly its isotope Promethium-147. The current production methods, such as irradiating uranium or thorium in nuclear reactors, are complex and expensive. Scientists are exploring alternative production techniques that could make Promethium more accessible for use in emerging technologies. Additionally, the storage of radioactive materials like Promethium presents significant challenges, as containment materials must be able to withstand the long-term decay of isotopes. Research into more durable storage solutions and radiation shielding technologies is ongoing, aimed at improving the safety and longevity of Promethium-containing devices.
Potential New Applications
As technology advances, the potential applications of Promethium are expanding, especially in fields like energy generation, medicine, and space exploration. In energy generation, Promethium’s radioactive properties could play a greater role in future nuclear power systems or compact power sources for remote locations. For example, Promethium-based radioisotope thermoelectric generators (RTGs) could see more widespread use in powering small, autonomous devices or in advanced nuclear reactors, where its ability to provide reliable, long-term power could be crucial.
In the medical field, research into the use of Promethium in targeted radiation therapies is exploring ways to use its radioactive isotopes to treat cancer more effectively. Its ability to emit beta radiation in a controlled manner makes it a candidate for new treatment methods, such as internal radiation therapies for localized tumors.
Space exploration, too, continues to offer new prospects for Promethium’s use. Given its ability to generate steady power over long durations, Promethium could play an even larger role in future space missions. As spacecraft and satellites travel further from the Sun, the need for reliable energy sources like Promethium-powered RTGs becomes more critical, particularly for deep-space missions where solar power is insufficient.
As advancements in production, storage, and application techniques continue, Promethium may play an increasingly significant role in these and other fields, making it an element to watch for its future potential.